Folding Paper with a Hydraulic Press
What happens when you ignore the math

There’s a well-known bit of folklore that you can only fold a sheet of paper six times. The principle is true, although the details are wrong: as you keep folding paper, it gets thicker (twice as thick each time) and so harder to fold, and more of the length of the paper is used up in the curves around each fold.
The real rule is given by Gallivan’sPaper Folding Theorem, which relates the length of the paper, its thickness, and the number of times it can be folded. Gallivan’s formula for the minimum length is
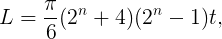
where t is the thickness of the paper and n is the number of folds.
Gallivan proved this while a junior in high school, and then demonstrated it by folding a sheet of toilet paper in half twelve times. Applied to a sheet of standard letter- or A4-sized paper, Gallivan’s formula gives you a maximum of just under or just over six folds, depending on the thickness of the paper, just as elementary-school folklore says.
But what happens if you try to do itanyway?
Here YouTube comes to the rescue, thanks to the Finnish operator ofThe Hydraulic Press Channel. As you may have guessed, this channel is dedicated to smashing things with a hydraulic press, and really this needs no further justification. So they decided to see what happens if you violate all the laws of mathematics by folding a sheet of A3 paper six times, and then fold it a seventh time using a press.
About two minutes and ten seconds in, the laws of physics assert themselves with a bang: with a loud noise, the paper is suddenly crushed, and out from the press comes a sheet of a flaky, limestone-like substance which once was paper.
What happened here? To be certain, I would need to repeat the experiment myself with a few more tools, like a thermocouple and an electron microscope. But I have a guess.
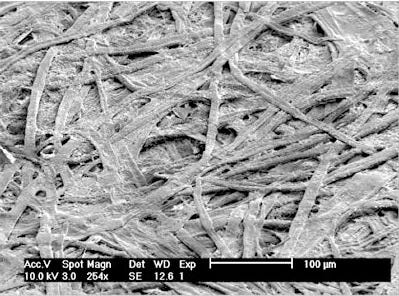
Paper is made by bleaching wood pulp to remove everything but the cellulose, then pressing and drying it into sheets. The result is a network of cellulose fibers, held together by “hydrogen bonds.”Under an electron microscope, it looks like this:
(In fact, you can even use this structure to “fingerprint” a piece of paper based on its microscopic shape!) Hydrogen bonds aren’t the ordinary chemical bonds you may have learned about in school: they’re what happens when two molecules next to each other have pointy bits sticking out (generally Hydrogen atoms hanging off the side, which is where they get their name). While free Hydrogen atoms have their (positively-charged) nucleus at the exact center of the (negatively-charged) electron cloud, the shape of the rest of the molecule pulls this electron cloud off to an angle. This means that from very close up, there’s a charge imbalance, and this imbalanced charge can pull at another imbalanced charge from a nearby molecule.
The resulting bond is very weak, only 1/1000th the strength of a normal chemical bond. But that makes these bonds incredibly important in biology, where it’s often very useful for a molecule to have a connection that can open and close without requiring enough power to rip the entire molecule apart.
When the piece of paper has been folded six times, there’s literally no way it can continue to fold at its current length. Gallivan’s Theorem tells us that to make it to a seventh fold, this sheet of paper would have to have been nearly a meter long — or phrased another way, for it to be crushed into a seventh fold, the paper will have to be stretched to over twice its original length.
There are thus two competing horizontal forces on the paper: the tension of the Hydrogen bonds holding the cellulose fibers together, and the tension of the paper being pulled by the force of the hydraulic press. At the same time, the paper is being compressed vertically (against its thickness).
Ultimately, something has to give. One thing that could happen is that the tension from the press wins, and the paper would simply tear at the fold. But apparently, something more is happening: the bonding of the cellulose into sheets is replaced by a bonding into a thick slab!
Both of these changes are happening because the force of the press is exceeding the bonding force of the Hydrogen bonds, so it’s not surprising that they happen at more or less the same time. What seems to be happening is that the amount of energy being transferred via the compression is finally enough to “melt” the bonds, and the paper momentarily ceases to be a sheet of cellulose fibers, and instead becomes a sort of three-dimensional cellulose soup. As soon as the tension is released, the compression of the paper continues very quickly, slamming the head of the press into the anvil, and the Hydrogen bonds can re-form.
But now, that slow and careful drying process which formed cellulose into sturdy sheets has been replaced by rapidly turning cellulose back into pulp, mashing it, and quickly re-drying it. So unsurprisingly, it isn’t very structurally sound, and it still has a lot of its old “sheet” structure, which is what makes it flaky and limestone-like.
To test this out properly, of course, we’d need to study it carefully: measure the pressure at which things happen (so that we can verify that the amount of energy being transferred is about enough to break Hydrogen bonds), measure the temperature of the system (so that we can see if there’s a sudden change, which we might expect), and most importantly, looking at the result under an electron microscope, so that we can see what the fibers look like before and after.
If anyone has these tools on hand, I think some important science needs to be done. And a new YouTube video has to be made.


No comments:
Post a Comment